Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-05-09 , DOI: 10.1016/j.seppur.2023.124033
Emma Feldt , Julia Järlebark , Vera Roth , Rasmus Svensson , Pontus K.G. Gustafsson , Nora Molander , Cristian Tunsu , Björn Wickman
|
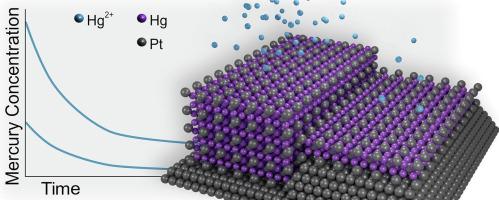
New and improved methods to remove toxic mercury from contaminated waters and waste streams are highly sought after. Recently, it was shown that electrochemical alloy formation of PtHg4 on a platinum surface with mercury ions from solution can be utilized for decontamination, with several advantages over conventional techniques. Herein, we examine the alloy formation process in more detail by mercury concentration measurements using inductively coupled plasma mass spectrometry in batch measurements as well as electrochemical quartz crystal microbalance analysis both in batch and in flowing water with initial mercury concentrations ranging from 0.25 to 75000 µg L−1 Hg2+. Results show that mercury is effectively removed from all solutions and the rate of alloy formation is constant over time, as well as for very thick layers of PtHg4. The apparent activation energy for the electrochemical alloy formation was determined to be 0.29 eV, with a reaction order in mercury ion concentration around 0.8. The obtained results give new insights that are vital in the assessment and further development of electrochemical alloy formation as a method for large scale mercury decontamination.
中文翻译:

用于汞净化的电化学 PtHg4 合金形成的温度和浓度依赖性
从受污染的水和废物流中去除有毒汞的新方法和改进方法备受追捧。最近,研究表明,与溶液中的汞离子在铂表面上形成电化学合金 PtHg 4可用于去污,与传统技术相比具有多项优势。在此,我们通过在批次测量中使用电感耦合等离子体质谱法测量汞浓度,以及在批次和初始汞浓度范围为 0.25 至 75000 µg·L 的流动水中进行电化学石英晶体微量天平分析,更详细地检查合金形成过程−1 Hg 2+. 结果表明,汞已从所有溶液中有效去除,并且合金形成速率随时间保持恒定,对于非常厚的 PtHg 4层也是如此。电化学合金形成的表观活化能被确定为 0.29 eV,汞离子浓度的反应级数约为 0.8。获得的结果提供了新的见解,这对于评估和进一步开发电化学合金形成作为大规模汞净化方法至关重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号