当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Acid-Promoted [2 + 2] Cycloadditions of Allenes and Ketenes: Versatile Methods for Natural Product Synthesis
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-08-04 , DOI: 10.1021/acs.accounts.3c00334
Renyu Guo 1 , M Kevin Brown 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-08-04 , DOI: 10.1021/acs.accounts.3c00334
Renyu Guo 1 , M Kevin Brown 1
Affiliation
|
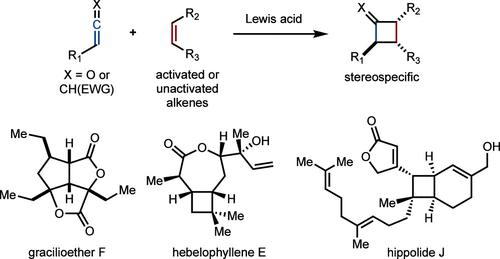
Cycloaddition reactions are an effective method to quickly build molecular complexity. As predicted by the Woodward–Hoffmann rules, concerted cycloadditions with alkenes allow for the constructions of all possible stereoisomers of product by use of either the Z or E geometry. While this feature of cycloadditions is widely used in, for example, [4 + 2] cycloadditions, translation to [2 + 2] cycloadditions is challenging because of the often stepwise and therefore stereoconvergent nature of these processes. Over the past decade, our lab has explored Lewis acid-promoted [2 + 2] cycloadditions of electron-deficient allenes or ketenes with alkenes. The concerted, asynchronous cycloadditions allow for the synthesis of various cyclobutanes with control of stereochemistry.
中文翻译:

路易斯酸促进的丙二烯和烯酮的 [2 + 2] 环加成:天然产物合成的多功能方法
环加成反应是快速构建分子复杂性的有效方法。正如伍德沃德-霍夫曼规则所预测的,与烯烃的协同环加成允许使用Z或E几何结构构建所有可能的产物立体异构体。虽然环加成的这一特征广泛用于例如[4 + 2]环加成,但由于这些过程通常是逐步的,因此具有立体收敛性质,因此转换为[2 + 2]环加成具有挑战性。在过去的十年中,我们的实验室探索了路易斯酸促进的缺电子丙二烯或烯酮与烯烃的[2 + 2]环加成反应。协同异步环加成允许在立体化学控制下合成各种环丁烷。
更新日期:2023-08-04
中文翻译:

路易斯酸促进的丙二烯和烯酮的 [2 + 2] 环加成:天然产物合成的多功能方法
环加成反应是快速构建分子复杂性的有效方法。正如伍德沃德-霍夫曼规则所预测的,与烯烃的协同环加成允许使用Z或E几何结构构建所有可能的产物立体异构体。虽然环加成的这一特征广泛用于例如[4 + 2]环加成,但由于这些过程通常是逐步的,因此具有立体收敛性质,因此转换为[2 + 2]环加成具有挑战性。在过去的十年中,我们的实验室探索了路易斯酸促进的缺电子丙二烯或烯酮与烯烃的[2 + 2]环加成反应。协同异步环加成允许在立体化学控制下合成各种环丁烷。

































 京公网安备 11010802027423号
京公网安备 11010802027423号