Abstract
Background
The plethysmography variability index (PVI) is a non-invasive, real-time, and automated parameter for evaluating fluid responsiveness, but it does not reliably predict fluid responsiveness during low tidal volume (VT) ventilation. We hypothesized that in a ‘tidal volume challenge’ with a transient increase in tidal volume from 6 to 8 ml Kg− 1, the changes in PVI could predict fluid responsiveness reliably.
Method
We performed a prospective interventional study in adult patients undergoing hepatobiliary or pancreatic tumor resections and receiving controlled low VT ventilation. The values for PVI, perfusion index, stroke volume variation, and stroke volume index (SVI) were recorded at baseline VT of 6 ml Kg− 1, 1 min after the VT challenge (8 ml Kg− 1), 1 min after VT 6 ml Kg− 1 reduced back again, and then 5 min after crystalloid fluid bolus 6 ml kg− 1 (actual body weight) administered over 10 min. The fluid responders were identified by SVI rise ≥ 10% after the fluid bolus.
Results
The area under the receiver operating characteristic curve for PVI value change (ΔPVI6–8) after increasing VT from 6 to 8 ml Kg− 1 was 0.86 (95% confidence interval, 0.76–0.96), P < 0.001, 95% sensitivity, 68% specificity, and with best cut-off value of absolute change (ΔPVI6–8) = 2.5%.
Conclusion
In hepatobiliary and pancreatic surgeries, tidal volume challenge improves the reliability of PVI for predicting fluid responsiveness and changes in PVI values obtained after tidal volume challenge are comparable to the changes in SVI.
Similar content being viewed by others
1 Introduction
Hepatobiliary and pancreatic surgeries have been associated with high mortality and morbidity rates, but the latest improvements in anesthesia and surgery management have significantly limited the operative hazards [1]. The management of patients undergoing these major surgeries in the perioperative period is frequently not at ease due to coexisting diseases or debilitation found in many patients as well as due to the potential for significant operative blood loss [2]. The liberal intravenous fluids administration may lead to deleterious pulmonary congestion, impaired wound healing, and acute kidney injury. On the other hand, intraoperative fluid restriction approach targeting a net balance of zero may impair perfusion of vital organs and lead to significantly higher rates of acute kidney injury, oliguria, and renal-replacement therapy [3]. Messina and colleagues reported no significant difference between the two approaches in the incidence of the postoperative major complications or mortality but they reported significant higher incidence of postoperative renal complication in the fluid restriction approach targeting a net balance of zero for elective abdominal surgery in a subgroup analysis [4]. During major hepatobiliary and pancreatic surgeries, intravascular volume expansion is continuously required, but the margin of error is rather narrow [5]. The intraoperative fluid administration is better to be individualized and customed for case by case requirements guided by valid and reliable dynamic index tools. The plethysmography variability index (PVI) is an automated, real-time, and non-invasive dynamic parameter with good potential to predict the fluid responsiveness. PVI estimates the respiratory changes gained by the pulse oximeter waveform [6, 7]. PVI is based on continuous calculations of the respirational alterations of the amplitude of the pulse oximetry waveform of mechanically ventilated patients. These respirational alterations produced by cardiopulmonary interactions, that are markedly affected by lung mechanics determinants as tidal volume (VT) values, could significantly influence PVI values [8]. It is presumed that VT ≥ 8 ml kg− 1 could significantly alter PVI values for certain patients groups [10]. Using low VT for mechanical ventilation is currently the preferred policy for safe lung protection at intensive care units (ICU) and during anesthesia conducts [10]. The ‘tidal volume challenge’ is proved to be a valid test that help to improve the reliability of dynamic indexes for predicting fluid responsiveness in patients receiving low VT ventilation by transiently increasing VT from 6 to 8 ml kg− 1 of predicted body weight (PBW) [11,12,13]. Up to our best knowledge, there are no studies that evaluated the potential of VT challenge to improve the reliability of PVI in predicting fluid responsiveness in patients receiving low VT ventilation. In this study, we investigated whether a transient increase in VT from 6 to 8 ml kg− 1 could improve the predictability of fluid responsiveness by using PVI values change in patients receiving low VT ventilation during hepatobiliary and pancreatic tumor resection surgeries.
2 Methods
We performed this prospective interventional study on adult patients undergoing hepatobiliary and pancreatic tumor resection surgeries after obtaining approval from the Ethical and Scientific Committee of Fayoum University Hospital (D154) and the National Liver Institute (0146/2018), Egypt. We received written informed consent from the patients or their surrogates to participate in this study. We registered this study at Clinicaltrials.gov (NCT03546179). The study was conducted at the National Liver Institute Hospital, Menoufia, Egypt. We studied patients aged ≥ 18 years undergoing open (non-laparoscopic) hepatobiliary or pancreatic tumor resection. Patients with preoperative cardiac arrhythmias, peripheral vascular disease, low left ventricular function (ejection fraction < 40%), significant valvular heart disease, metastasis, irresectable tumor, massive blood loss, or fulminant hepatic failure were excluded from this study.
On the day of surgery, intravenous access was obtained. Demographic and anthropometric data including age, sex, height, actual body weight (ABW), PBW, body mass index (BMI), body surface area (BSA), smoking history, comorbid diseases, and use of vasopressors were recorded. All patients were monitored using 5-lead electrocardiography, non-invasive blood pressure monitoring, and pulse oximetry. General anesthesia was induced with propofol (2 mg kg− 1 IV), fentanyl (3 µg kg− 1 IV) and rocuronium (0.6 mg kg− 1 IV). Anesthesia was maintained with sevoflurane (1.5–3%) and 10 mg rocuronium / 30 min to maintain adequate muscle relaxation. Mechanical ventilation commenced initially with volume-controlled ventilation mode, VT = 6 ml Kg− 1 (PBW), Positive end-expiratory pressure (PEEP) = 3–5 cmH2O, respiratory rate = 10–12 breath/minute, and a fraction of inspired oxygen (FiO2) = 0.5–0.7 (Oxygen air mixture) to achieve and maintain a peripheral oxygen saturation (SPO2) ≥ 96%. The PBW (kg) was calculated as follows: X + 0.91[height (cm) − 152.4]; (X = 50 for men and = 45.5 for women). GE Avance® CS 2 anesthesia machines (GE Healthcare, Madison, WI, USA).
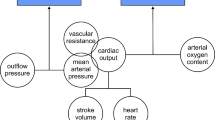
A 7 F triple-lumen central venous catheter was inserted in the right internal jugular vein with real-time ultrasound guidance and complete aseptic precaution. A pressure transducer was connected to the 16-gauge lumine to continuously monitor the central venous pressure (CVP). The left radial artery was cannulated for measuring mean arterial pressure (MAP) invasively. The pressure transducers for both CVP and invasive arterial blood pressure were placed on the midaxillary line, fixed to the operating table to keep the sensor at the atrial level, and zeroed to atmospheric pressure. An indwelling urinary bladder catheter was inserted to monitor urinary output. Head and extremity wrap and forced warming system were applied to maintain body temperature. A trans-esophageal doppler probe (Cardio QP EDM™; Deltex Medical, Chichester, UK) was greased with a lubricating gel and passed nasally into the mid-esophagus until the aortic blood flow signals were best identified. The Masimo Pulse Co-Oximeter probe (Masimo SET Rainbow R2-25r and R225a; Masimo Corp., Irvine, CA, USA) was placed on the index finger of patients and covered to avoid light interference. The Stroke volume (SV) and stroke volume variation (SVV) were measured directly by the trans-esophageal doppler probe. The stroke volume index (SVI) was calculated by dividing the value of SV by BSA (obtained by Cardio QP EDM™ monitor after providing age, sex, weight, and height of the patient). The PVI and Perfusion index (PI) values were reported by the aid of The Masimo Pulse Co-Oximeter monitor. Throughout surgery, packed red blood cells (300 ml) were transfused when the hematocrit level was < 25%. Fresh frozen plasma (200 ml) was administrated when the fibrinogen level was < 2 g dl− 1 or the international normalized ratio was > 2. Patients were extubated either in the operating room or postoperatively in the ICU after completion of surgical intervention.
3 Intervention protocol (Fig. 1)
After the tumor resection phase, baseline hemodynamic variables including heart rate (HR), MAP, CVP, SVI, PVI, PI, and SVV with a 6 ml kg− 1 VT ventilation (baseline 1 time-point) were recorded. After baseline measurement, VT was increased to 8 ml kg− 1 PBW for 1 min (after VT challenge time-point), and the hemodynamic variables were obtained. The VT has been reduced back to 6 ml kg− 1 PBW again and after 1 min, the hemodynamic measurements were recorded (baseline 2 time-point). After these hemodynamic measurements, volume expansion was performed for 10 min using an infusion of a balanced crystalloid solution (6 ml kg− 1 ABW). The same hemodynamic parameters were measured under ventilation with a VT of 6 ml kg− 1 five minutes after fluid bolus administration (after fluid bolus time-point). Then, the absolute change (ΔPVI6–8) (PVI after − PVI before VT challenge) and percentage change (%ΔPVI6–8) (PVI after − PVI before VT challenge / PVI before VT challenge) between the PVI at 6 ml kg− 1 of PBW (PVI6) and that at 8 ml kg− 1 of PBW (PVI8) (i.e., after performing a VT challenge) were calculated. The percentage change in SVI, according to volume loading, was used as the principal indicator of fluid responsiveness. Responders or non-responders were determined when the increase in SVI was ≥ 10% or < 10% after fluid bolus, respectively [14]. No more than two VT challenges can be performed in any patient. Doses of vasoactive medications, if used, and PEEP were kept constant one minute before and during the four time-points of data acquisition. We defined the primary outcome as the best cut-off value of ΔPVI6–8 in hepatobiliary or pancreatic surgeries using a VT challenge. The grey zone was defined as a range between a low cutoff value of ΔPVI6–8 that included 90% of fluid responders and a high cutoff value of ΔPVI6–8 that included 90% of fluid non-responders [15, 16].
The secondary outcomes were the evaluation of the hemodynamic variables (HR, MAP, CVP, SVI, PVI, PI, and SVV) at four time-points (VT 6 ml/kg baseline 1, VT 8 ml/Kg after VT challenge, VT 6 ml/Kg baseline 2, and after fluid bolus administration) for fluid responders and non-responders. Additionally, calculation of the area under the curve (AUC), detection of sensitivity, specificity, and best cutoff value of PVI6, PVI8, ΔPVI6–8, %ΔPVI6–8, and other statistically significant differences of the other measured hemodynamic variables of fluid responsiveness.
4 Sample size calculation and statistical analysis
The online Statstodo application (www.statstodo.com) was utilized to determine the sample size prerequisite for assessing two receiver operating characteristics (ROC) curves with anticipated AUC of 0.65 (PVI6) and 0.90 (ΔPVI6–8), presuming an α error = 0.05 and a power = 90%. A minimum of 40 patients were considered necessary to identify an AUC difference of 0.25 when assuming equal numbers of fluid responders and non-responders [17]. As the data loss was likely to be within 20%, 48 patients have to be enrolled in this study.
The SPSS software Version 21.0 (IBM, Armonk, NY, USA) was used to perform statistical analysis. Data were presented as mean ± standard deviation (SD), median [interquartile range (IQR) (range)], or percentage (%). Distribution normality was assessed using the Shapiro-Wilk test. The changes in continuous variables from 6 to 8 mg kg− 1 PBW (after VT challenge) and the changes before and after fluid bolus were compared using the paired t-test or Wilcoxon signed rank-sum test. Group comparisons between responders and non-responders were performed using the independent t-test or Mann–Whitney U test. Categorical variables were analyzed using the chi-square test or Fisher exact test when indicated. To test the abilities of the dynamic preload indices to predict fluid responsiveness, the AUCs of responders were calculated and compared using the Hanley–McNeil test (AUC = 0.5, a useless test with no possible prediction; AUC = 0.6–0.69, a test with poor predictability; AUC = 0.7–0.79, a fair test; AUC = 0.8–0.89, a test with good predictability; AUC = 0.9–0.99, an excellent test; AUC = 1.0, a perfect test with the best possible prediction) [18]. An optimal threshold value was determined for each variable to maximize the Youden index [sensitivity + (specificity − 1)] [19].
5 Results
The patients in the current study were enrolled between August 2018 and February 2020. Sixty-four consecutive hepatobiliary or pancreatic tumour resection patients were considered eligible for inclusion. There were three patients excluded before and thirteen patients excluded after the enrolment. (Fig. 2) Finally, forty-eight patients were analysed, twenty patients (41.7%) were fluid responders (SVI rise by more than or equal 10% after crystalloid fluid bolus 6 ml Kg − 1 ABW) while twenty-eight patients (58.3%) were fluid non-responders (SVI rise by less than 10%). Each enrolled patient experienced only VT challenge once. Demographic characteristics (age, sex, weight, height, PBW, BMI, and BSA), comorbidities, surgical procedures, and vasopressors use were comparable between responders and non-responders. (Table 1) All the hemodynamic values of responders and non-responders before fluid bolus administration were comparable at Baseline 2 time-point. (Table 2) The fluid bolus administration significantly increased SVI in responders from mean ± SD 41.3 ± 9.9 ml. beat− 1.m− 2 to mean ± SD 49.6 ± 13 ml. beat− 1.m− 2, P < 0.001 and there was statistically significant difference between responders mean ± SD 49.6 ± 13 ml. beat− 1.m− 2 and non-responders mean ± SD 42 ± 10 ml. beat− 1.m− 2, P = 0.03 after fluid bolus. (Table 2) The HR and MAP values were comparable before and after the fluid bolus for responders and non- responders. The CVP values were comparable before and after the fluid bolus for fluid responders but there was statistically significant rise of CVP values for non-responders after the fluid bolus as compared to the values before the fluid bolus, P = 0.02. This statistical significance was of trivial clinical impact. (Table 2) There were statistically significant differences of PI values after fluid bolus for responders, P = 0.03 and non- responders, P = 0.01 but the differences were comparable between responders and non- responders before, P = 0.14 and after, P = 0.11 the fluid bolus administration. (Table 2) For SVV values, the changes were comparable between responders and non-responders before and after the fluid bolus administration. (Table 2) All the hemodynamic variables of responders and non-responders before the VT challenge were comparable at Baseline 1 time-point. For the values of HR, MAP, CVP, SVI, PI, and SVV, there were no statistically significant differences between responders and non-responders after tidal volume challenge time-point. Additionally, there were no statistically significant differences of the previously mentioned variables when comparing these values before and after applying VT challenge for both responders and non-responders. (Table 2) The VT challenge increase PVI in fluid responders from median [IQR (range)] 9.5% [9–12 (4–29)] at VT 6 ml Kg− 1 baseline 1 time-point to 18% [12–19 (7–35)] at VT 8 ml Kg− 1 after VT challenge, P < 0.001. This difference was not significant in non-responders. (Table 2) The AUC of PVI6 was 0.49 (95% confidence interval (CI), 0.33–0.66), P = 0.99 showing non-significant prediction of fluid responsiveness. The AUC of PVI8 was 0.79 (95% CI, 0.66–0.92), P = 0.001 showing a sensitivity of 85% and a specificity of 61% for a best cutoff value of 11.5%. The AUC of ΔPVI6–8 was 0.86 (95% CI, 0.76–0.96), P < 0.001 showing a sensitivity of 95% and a specificity of 68% for a best cutoff value of a 2.5%. (Table 3; Fig. 3) The ΔPVI6–8 values of four enrolled patients (8.3%) were within the inconclusive grey-zone range of the test (1–6%). The fluid bolus cause PVI values reduction in fluid responders from median [IQR (range)] 11.5 [9.2–16 (5–29)] at VT 6 ml Kg− 1 baseline 2 time-point to 9 [5.5–10 (3–17)] at VT 6 ml Kg− 1 after fluid bolus, P < 0.001. This difference was not significant in non-responders, P = 0.12. The differences between fluid responders and non-responders were comparable at the same time-points. (Table 2) The AUC of change of PVI after fluid bolus was 0.78 (95% CI, 0.65–0.92), P = 0.001 showing a sensitivity of 70% and a specificity of 79% with a best cutoff value of 2%. (Table 3; Fig. 3)
6 Discussion
This study reveals the ability of VT challenge to enhance the PVI potential to predict fluid responsiveness for non-laparoscopic hepatobiliary or pancreatic tumors resection procedures in mechanically ventilated patients under general anesthesia. The absolute change in PVI (ΔPVI6–8) reliably predicts fluid responsiveness with a cutoff value of 2.5% with excellent prediction of most of fluid responders, 95% sensitivity and with uncertain prediction of fluid non-responders (could miss one in every three fluid non-responders) (30% of non-responders recognized imperfectly as fluid responders), specificity 68%. The PVI6 has no potential to predict fluid responsiveness efficiently and the inconclusive grey-zone range of ΔPVI6–8 lies between 1% and 6% indicate uncertain predictive value. It is a quite wide range, but the total number of patients involved within the inconclusive grey-zone was small. There is associated intrathoracic pressure rise with providing mechanical ventilation using higher VT of 8 ml kg− 1. The subsequent cardiopulmonary interactions significantly lower the cardiac preload status in certain groups of patients (fluid responders). This effect is masked when using a low VT ventilation that minimizes these cardiopulmonary interactions and makes it difficult to differentiate between the fluid responders and non-responders. Transient increase of VT during mechanical ventilation uncover this effect and help to identify the fluid responders [11]. The inherit limitations of PVI reliability of prediction of fluid responsiveness for spontaneously breathing patients, marked arrythmias, increased intraabdominal pressure [20, 21] decreased lung compliance, and right ventricular dysfunctions [21,22,23,24] might be attributed to dysregulated respiratory alterations and cardiopulmonary interactions in such conditions. Cannesson and colleagues concluded that PVI predicts fluid responsiveness efficiently in a group of patients under mechanical ventilation after anesthesia induction for coronary artery bypass grafting. They used VT 8–10 ml Kg− 1 continually throughout data acquisition time and they found PVI was reduced from 14 to 9% after 500 ml hetastarch 6% fluid bolus. They determined a cutoff value of PVI = 14% for fluid responsiveness with 81% sensitivity and 100% specificity. They highlighted a significant relationship between PVI values change and cardiac index variations after the fluid bolus. They waited equilibrium for four minutes (3 min after volume expansion and one minute after no stimulation) after fluid bolus to collect the hemodynamic variables to assess the effects of volume expansion on cardiac output and other hemodynamic variables [22]. The findings of the current trial are consistent with that of Desebbe and colleagues. They showed a substantial differences of PVI values when using VT ≥ 8 ml kg− 1 as compared with 6 ml Kg− 1 in prediction of hemodynamic instability before applying sudden positive PEEP value of 10 cmH2O with acceptable sensitivity and specificity in a group of patients who were sedated and mechanically ventilated postoperatively [25]. They demonstrated that differences of PVI values obtained during lower VT ventilation (6 ml kg− 1) could not predict the hemodynamic instability efficiently following sudden PEEP application [25]. In the same context, Desgranges and colleagues used 8 ml Kg− 1 VT for mechanical ventilation after anesthesia induction to compare three pulse oximeter sensors sites (the finger, ear, and forehead). They reported that the three sensor sites were able to predict the fluid responsiveness efficiently and comparably between the three sites [26]. The VT challenge during mechanical ventilation under anesthesia was used efficiently to improve the capabilities of the dynamic indices for fluid responsiveness prediction [12, 13]. Messina and colleagues applied tidal volume challenge in a group of neurosurgical patients in prone position. They reported that the VT challenge using 8 ml Kg− 1 VT could increase the predictability of the pulse pressure variation (PPV) and SVV for fluid responsiveness with favorable sensitivity and specificity to avoid unnecessary intravenous fluid administration of such critical group of patients [13]. Their findings were emphasized on other cohort of elective neurosurgical patient on supine position [12]. In contrast to the findings of Messina and colleagues, we found that VT challenge could not improve the reliability of SVV to predict fluid responsiveness as there were insignificant differences between the responders and non-responders at all time-points of assessment in addition to that all comparisons before, during, and after VT challenge were comparable. This discrepancy could be attributed to the different methods of measurement of SVV or different cohort of patients between the current trial and that trials of Messina and colleagues [12, 13]. In contrast to the findings of the current trial, Myatra and colleagues reported significant difference of SVV values after VT challenge 8 ml Kg− 1 with sensitivity 75% and specificity 76% in a group of ICU patients who were sedated, mechanically ventilated, diagnosed with septic shock, and with possibly defective lung compliance. They obtained SVV values by transpulmonary thermodilution method [11]. The values of HR and MAP were comparable before and after VT challenge, before and after fluid bolus, and between fluid responders and non-responders at all time points of assessment. These findings emphasized the poor ability of these hemodynamic variables to differentiate between fluid responders and non-responders. There was statistically significant difference for CVP values before and after the fluid bolus for the fluid non-responders only but of minute clinical effect. This difference may highlight that an increase in preload in fluid non-responders will not augment cardiac output and could impair the cardiac function by increasing the preload of right ventricle. CVP values before and after VT challenge for both fluid responders and non-responders were comparable and the CVP values before and after fluid bolus for responders were comparable denoting the inability of this parameter to predict fluid responsiveness efficiently. The current study utilized the independent SVI variables as a cardiac output surrogate to differentiate between fluid responders and non-responders. It is calculated by dividing the SV value (obtained by trans-esophageal doppler probe) by BSA. It is not as accurate as the values obtained by the more invasive pulmonary artery catheter with standard thermodilution method [22]. but probably it is more reliable than the non-calibrated peripheral pulse contour analysis values of SV [11]. or values determined by the non-invasive bioreactance methods [27]. The minimally invasive trans-esophageal doppler measure the stroke distance (flow velocity multiplied by flow time) of the descending part of thoracic aorta. SV is calculated as Stroke distance multiplied by aortic cross-section area (which is an area of a circle πr2). The radius of the descending aorta (which is used to calculate aortic cross-section area) is derived from published nomograms based on age, sex, weight, and height (Deltex, West Sussex, England, www.deltexmedical.com) [28]. The resulting values of SV were then compensated (by 30%) for the fraction of blood flow that circumvented to coronaries and aortic arch branches (cerebral and upper limbs blood flow) [28,29,30]. The 10% cutoff value to differentiate fluid responders and non-responders was utilized before for the same aim by Messina and colleagues who obtained the SV values by radial artery waveform analysis [12]. The values of PI before and after the VT challenge for both fluid responders and non-responders were comparable. On the other hand, there were statistically significant differences of PI values before and after fluid bolus for both the fluid responders, P = 0.03 and non-responders, P = 0.01. This effect could be interpreted as PI is not a good predictor of fluid responsiveness, but fluid bolus administration could increase the values of PI in the fluid responders and non-responders similarly without consequent impact on the cardiac output. The PI value changes may possibly suggest intravascular volume status changes that not based on respiratory alterations and cardiopulmonary interactions. These findings go in line with the findings of Cannesson and colleagues [22].
7 Study limitations
The current trial is a non-randomized study recruiting 64 successive patients for hepatobiliary and pancreatic tumor resection. Its findings should be manipulated and interpreted carefully as data acquisition done under rigorous controlled circumstances in selected group of patients. We did unplanned additional patients’ exclusion before and after enrolment process to keep this non-randomized cohort of patients homogenous under same standard circumstances to minimize the risk of bias. Before enrollment, one laparoscopic hepatobiliary tumor resection case was excluded as the pneumoperitoneum could increase intraabdominal pressure with subsequent affection of respiratory alteration and heart-lung interactions that could interfere with PVI values changes and accuracy of fluid responsiveness prediction. Tissue dissection, third space fluid losses, fluid shifts, and intraoperative blood loss were supposed to be less in laparoscopic surgery than in open type procedures. An additional factor to exclude this case is frequent position changing (head up and down positions). There were four cases of irresectable tumors, and one case of undiagnosed metastasis that were excluded from the study after enrolment as these inoperable cases were subjected to less negligible tissue dissection, fluid shifts, and blood loss than the patients undergone complete tumor resection who may require more volume expansion, blood transfusion, or vasoactive drugs administrations. There was one case of massive blood loss excluded after enrollment as the patient needed massive blood transfusion with high dose vasopressors and experienced marked hemodynamic instability that precluded protocol completion. The five minutes interval between completion of crystalloid fluid bolus (over ten minutes) and acquisition of hemodynamic variable after fluid bolus was relatively long. This delay might result in fading and possibly underestimating the assumed clinical effects of volume expansion and the final outcome.
8 Conclusion
For optimizing intraoperative fluid administration in hepatobiliary and pancreatic surgeries, VT challenge improves the prediction accuracy of PVI to reliably identify fluid responders and non-responders as changes in PVI values obtained by transient increase of VT to 8 ml Kg− 1 are more predictive than PVI values that are reported during low tidal volume ventilation (6 ml Kg− 1).
References
Tympa A, Theodoraki K, Tsaroucha A et al (2012) Anesthetic considerations in hepatectomies under hepatic vascular control. HPB Surg 2012:12, Article ID 720754. https://doi.org/10.1155/2012/720754
Ramsay M. Hepatic physiology & anesthesia. In: Butterworth JF, Mackey DC, Wasnick JD, editors. Morgan & Mikhail’s clinical anesthesiology. 6th ed. Eds. New York: McGraw-Hill Education. 2018. pp. 1164–92.
Myles PS, Bellomo R, Corcoran T et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018; 378(24):2263–2274. https://doi.org/10.1056/NEJMoa1801601
Messina A, Robba C, Calabrò L et al. (2021) Perioperative liberal versus restrictive fluid strategies and postoperative outcomes: a systematic review and metanalysis on randomised-controlled trials in major abdominal elective surgery. Crit Care 25, 205 (2021):1–13. https://doi.org/10.1186/s13054-021-03629-y
Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. (2004) The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg 187(3):398–402. https://doi.org/10.1016/j.amjsurg.2003.12.001
Konur H, Erdogan Kayhan G, Toprak HI, Bucak N, Aydogan MS, Yologlu S, Durmus M, Yılmaz S (2016) Evaluation of pleth variability index as a predictor of fluid responsiveness during orthotopic liver transplantation. Kaohsiung J Med Sci 32(7):373–80. https://doi.org/10.1016/j.kjms.2016.05.014
Lee HC, Tsai YF, Tsai HI, Chung PCH, Yu HP, Lee WC, Lin CC (2016) Pulse oximeter–derived pleth variability index is a reliable indicator of cardiac preload in patients undergoing liver transplantation. Transplant Proc 48(4):1055–1058. https://doi.org/10.1016/j.transproceed.2015.12.106
Desgranges FP, Evain JN, Pereira de Souza Neto E, Raphael D, Desebbe O, Chassard D (2016) Does the plethysmographic variability index predict fluid responsiveness in mechanically ventilated children? A meta-analysis. Br J Anaesth 117(3):409–10. https://doi.org/10.1093/bja/aew245
Antonsen LP, Kirkebøen KA (2012) Evaluation of fluid responsiveness: is photoplethysmography a noninvasive alternative? Anesthesiol Res Pract 617380:1–10. https://doi.org/10.1155/2012/617380
Futier E, Jean-Michel Constantin JM, Catherine Paugam-Burtz C et al (2013) A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369(5):428–37. https://doi.org/10.1056/NEJMoa1301082
Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL (2017) The changes in pulse pressure variation or stroke volume variation after a ‘tidal volume challenge’ reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med 45(3):415–21. https://doi.org/10.1097/CCM.0000000000002183
Messina A, Montagnini C, Cammarota G et al (2019) Tidal volume challenge to predict fluid responsiveness in the operating room: an observational study. Eur J Anaesthesiol 36(8):583–91. https://doi.org/10.1097/EJA.0000000000000998
Messina A, Montagnini C, Cammarota G et al (2020) Assessment of fluid responsiveness in prone neurosurgical patients undergoing protective ventilation: role of dynamic indices, tidal volume challenge, and end-expiratory occlusion test. Anesth Analg 130(3):752–61. https://doi.org/10.1213/ANE.0000000000004494
Min JJ, Gil NS, Lee JH, Ryu DK, Kim CS, Lee SM (2017) Predictor of fluid responsiveness in the ‘grey zone’: augmented pulse pressure variation through a temporary increase in tidal volume. Br J Anaesth 119(1):50–6. https://doi.org/10.1093/bja/aex074
Cannesson M, Le Manach Y, Hofer CK et al (2011) Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 115(2):231–41. https://doi.org/10.1097/ALN.0b013e318225b80a
Biais M, Larghi M, Henriot J, de Courson H, Sesay M, Nouette-Gaulain K (2017) End-expiratory occlusion test predicts fluid responsiveness in patients with protective ventilation in the operating room. Anesth Analg 125(6):1889–95. https://doi.org/10.1213/ANE.0000000000002322
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143(1):29–36. https://doi.org/10.1148/radiology.143.1.7063747
Espinoza Ríos J, Huerta-Mercado Tenorio J, Huerta-Mercado Tenorio J et al (2009) Prospective validation of the Rockall Scoring System in patients with upper gastrointestinal bleeding in Cayetano Heredia Hospital Lima- Peru. Rev Gastroenterol Peru 29(2):111–7.
Ray P, Le Manach Y, Riou B, Houle TT (2010) Statistical evaluation of a biomarker. Anesthesiology 112(4):1023–40. https://doi.org/10.1097/ALN.0b013e3181d47604
Marik PE, Monnet X, Teboul JL (2011) Hemodynamic parameters to guide fluid therapy. Ann Intens Care 1(1):1–9. https://doi.org/10.1186/2110-5820-1-1
Pinsky MR (2015) Functional hemodynamic monitoring. Crit Care Clin 31(1):89–111. https://doi.org/10.1016/j.ccc.2014.08.005
Cannesson M, Desebbe O, Rosamel P et al (2008) Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth 101(2):200–6. https://doi.org/10.1093/bja/aen133
Shi R, Monnet X, Teboul JL (2020) Parameters of fluid responsiveness. Curr Opin Crit Care 26(3):319–26. https://doi.org/10.1097/MCC.0000000000000723
Monnet X, Rienzo M, Osman D et al (2005) Esophageal doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med 31(9):1195–201. https://doi.org/10.1007/s00134-005-2731-0
Desebbe O, Boucau C, Farhat F, Bastien O, Lehot JJ, Cannesson M (2010) The ability of pleth variability index to predict the hemodynamic effects of positive end-expiratory pressure in mechanically ventilated patients under general anesthesia. Anesth Analg 110(3):792–8. https://doi.org/10.1213/ANE.0b013e3181cd6d06
Desgranges FP, Desebbe O, Ghazouani A et al (2011) Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. Br J Anaesth 107(3):329–35. https://doi.org/10.1093/bja/aer165
Vergnaud E, Vidal C, Verchère J et al (2015) Stroke volume variation and indexed stroke volume measured using bioreactance predict fluid responsiveness in postoperative children. Br J Anaesth 114(1):103–9. https://doi.org/10.1093/bja/aeu361
Funk DJ, Moretti EW, Gan TJ (2009) Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg 108(3):887–97. https://doi.org/10.1213/ANE.0B013E31818FFD99
King SL, Lim MS (2004) The use of the oesophageal doppler monitor in the intensive care unit. Crit Care Resusc 6(2):113–22.
Archer TL, Funk DJ, Moretti E, Gan TJ (2009) Stroke volume calculation by esophageal doppler integrates velocity over time and multiplies this “area under the curve” by the cross sectional area of the aorta. Anesth Analg 109(3):996. https://doi.org/10.1213/ANE.0B013E3181AE901C
Acknowledgements
The authors thank the anesthesia staff and technicians of the National Liver Institute for their cooperation during this study.
Funding
The authors declared that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Yasser Salem Mostafa Mahmoud prepared the materials, collected the data, and shared in data analysis. Hany Mahmoud Yassin Moussa wrote the final drafts of the manuscript in addition to sharing in data analysis. All authors commented on and approved the previous and final versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was approved by the Ethical and Scientific Committee of Fayoum University Hospital and the National Liver Institute according to Helsinki declaration 1964 and later protocols.
Consent to participate
Informed consent was obtained from all individual participants included in the study or their surrogates.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- ABW
-
Actual body weight.
- AUC
-
area under the ROC curve.
- CI
-
cardiac index.
- CVP
-
central venous pressure.
- FiO2
-
fraction of inspired oxygen.
- HR
-
heart rate.
- ICU
-
intensive care unit.
- IQR
-
interquartile range.
- MAP
-
mean arterial pressure.
- PBW
-
predicted body weight.
- PEEP
-
Positive end-expiratory pressure.
- PPV
-
pulse pressure variation.
- ROC
-
receiver operating characteristic.
- SD
-
standard deviation.
- SPO2
-
Peripheral oxygen saturation.
- SV
-
stroke volume.
- SVI
-
stroke volume index.
- SVV
-
stroke volume variation.
- VT
-
tidal volume.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Botros, J.M., Salem, Y., Khalil, M. et al. Effects of tidal volume challenge on the reliability of plethysmography variability index in hepatobiliary and pancreatic surgeries: a prospective interventional study. J Clin Monit Comput 37, 1275–1285 (2023). https://doi.org/10.1007/s10877-023-00977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-00977-8